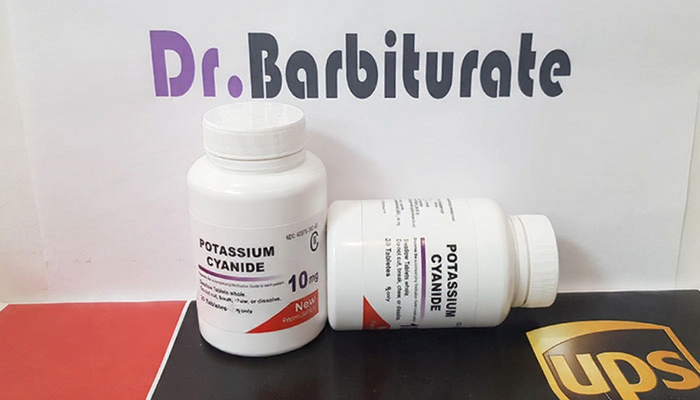
Buy POTASSIUM CYANIDE TABLETS online in the UK and Europe
Price range: £325.00 through £650.00
Description
Potassium cyanide (KCN) is a highly toxic chemical compound with a rich and complex history that spans various fields, including medicine, industry, and even criminal activity. Initially isolated in the early 19th century, this potent binary compound has played significant roles in processes such as gold extraction and organic synthesis, while also garnering notoriety for its association with infamous crimes and warfare. As regulations surrounding its use have evolved, the understanding of potassium cyanide’s properties and its potential risks has become increasingly important. This article delves into the history and development of potassium cyanide as a chemical agent, examining its chemical characteristics, industrial applications, and the regulatory frameworks shaping its use today, as well as considering future perspectives in safety and research.
Introduction to Potassium Cyanide
Definition and Chemical Composition
Potassium cyanide (KCN) is a potent chemical compound consisting of potassium (K), carbon (C), and nitrogen (N). In its crystalline form, it resembles white granules or powder that can be surprisingly deceptive in appearance—much like a sugar glider, cute but potentially deadly. With a cyanide ion (CN⁻) at its core, KCN is primarily known for its high toxicity, but it also has a variety of industrial applications that have shaped its historical narrative.
Overview of Its Historical Significance
The journey of potassium cyanide is a rollercoaster of intrigue, ranging from early industrial applications to its unfortunate associations with criminal activity. Discovered in the late 18th century, KCN quickly made headlines—not just for its lethal capabilities but also for its role in the advancement of various industries such as mining and electroplating. Its historical significance is a tapestry woven with threads of both scientific achievement and cautionary tales, leaving us to ponder: is it a hero or a villain in the world of chemistry?
Early Discoveries and Uses of Potassium Cyanide
First Isolation of Cyanide Compounds
The first known isolation of cyanide compounds dates back to the late 1700s when Swedish chemist Carl Wilhelm Scheele took the plunge into the world of chemical experimentation. Scheele’s reaction with potassium carbonate and sulfuric acid led to the birth of potassium cyanide, making him the unlikely father of this notorious compound. His discovery was the first step in a long, tumultuous love affair between humanity and cyanide—a relationship marked by both innovation and caution.
Initial Applications in Medicine and Mining
In the early stages, potassium cyanide found its way into the medical cabinet, primarily as a treatment for various ailments, including syphilis. However, this approach fizzled out faster than a soda left open for too long due to its toxic nature. Shortly thereafter, the compound made a name for itself in the mining industry, particularly in gold extraction, where it was used to dissolve gold from ores. It was the golden age of cyanide—literally—turning rocks into riches, one toxic reaction at a time.
Chemical Properties and Production Methods
Physical and Chemical Characteristics
Potassium cyanide possesses some striking physical and chemical properties. It boasts a white crystalline structure, highly soluble in water, which is just as inviting as it sounds. KCN’s melting point is relatively low, at around 634 °C (1173 °F), making it a swift operator in chemical reactions. However, its most notable characteristic is its toxicity; it can inhibit cellular respiration by blocking the enzyme cytochrome c oxidase, leading to asphyxiation. In short, this seemingly innocuous crystal is nature’s reminder that looks can be deceiving.
Synthesis Techniques and Industrial Processes
The synthesis of potassium cyanide can be achieved through several methods, but one of the most common is the reaction of sodium cyanide with potassium carbonate. This method, while effective, is often performed under controlled conditions due to the volatility of cyanide compounds. Industrially, KCN is produced in large quantities, primarily for use in mining and electroplating, where precise production techniques are paramount to ensure both efficiency and safety. Just like an overzealous baker, the chemistry must be just right to avoid any unwanted ‘explosions’—or worse.
Potassium Cyanide in Industrial Applications
Role in Gold Extraction and Electroplating
Potassium cyanide has carved out a significant niche in gold extraction, thanks to its ability to form complexes with gold ions in a process known as cyanidation. This technique has revolutionized the mining industry, allowing for the extraction of gold from low-grade ores that would otherwise be left untouched. Moreover, KCN plays a vital role in electroplating, where it is used to deposit a thin layer of gold onto various substrates, giving everything from jewelry to electronics a touch of luxe. Just don’t try to taste it—gold may be precious, but KCN is not.
Use in Organic Synthesis and Pharmaceuticals
Beyond the glimmer of gold, potassium cyanide is a key player in organic synthesis and pharmaceuticals. It’s a valuable reagent in the production of various organic compounds, including amino acids and pharmaceuticals, showcasing its versatility beyond just being a toxic menace. However, its pharmaceutical applications are met with caution; safety protocols in labs are tighter than a drum. When used correctly, potassium cyanide can help pave the way for advancements in science and medicine, proving that even the most dangerous substances can have redeeming qualities—if handled with care.
Historical Context: Potassium Cyanide in Warfare and Crime
Usage in Historical Conflicts
Potassium cyanide, often associated with dramatic plot twists in spy novels, has a less glamorous history in actual warfare. First synthesized in the 19th century, it found its way into military arsenals during World War I, touted for its potential lethality. While never widely used as a chemical weapon, its presence in the battlefield raised eyebrows and alarm, paving the way for stricter regulations on chemical warfare. Soldiers weren’t just battling enemy forces; they grappled with invisible threats lurking in their own ranks.
Infamous Crimes and Notable Cases
If potassium cyanide had a résumé, it would highlight a few infamous headlines. From high-profile poisoning cases to sinister acts of vengeance, this chemical compound has made its mark in criminal history. One particularly notable case involved the tragic and theatrical death of the boxer “Kid” McCoy, where cyanide left a lasting legacy of suspicion and intrigue. Its ability to deliver an untimely end swiftly and effectively has made it a weapon of choice for nefarious plots—both in reality and in our favorite crime dramas.
Regulatory Changes and Safety Protocols
Legislation Governing the Use of Potassium Cyanide
With great power comes great responsibility—or so the saying goes, and potassium cyanide is no exception. Following its historical misuse, governments worldwide began implementing strict regulations. The Chemical Facility Anti-Terrorism Standards in the U.S. and various international treaties aim to control the manufacture, sale, and storage of this deadly substance. It’s a tightrope walk between industrial use and safety, and legislation continues to evolve as we learn more about its risks.
Safety Measures and Handling Procedures
Handling potassium cyanide might not be on your résumé, but if it were, you’d need to brush up on safety protocols. Industries dealing with this chemical are mandated to implement rigorous training and safety measures. Think gloves, goggles, and a full hazmat suit—everything short of a superhero cape. The mantra is clear: prevention is key. Facilities are required to have spill containment systems and emergency response plans to ensure that mishaps are more of a plot twist than a disaster.
Modern Uses and Research on Potassium Cyanide
Current Trends in Industry and Research
Despite its dark past, potassium cyanide isn’t just a relic of history. In modern industries, it plays a crucial role in gold extraction, enabling miners to separate precious metals from their ores efficiently. Researchers are probing into its chemical properties, eyeing potential applications in everything from pharmaceuticals to agriculture. While that might sound like a leap, innovation often springs from the most unexpected places—sort of like finding a golden nugget in a pile of rocks.
Alternatives and Innovations in Cyanide Use
While potassium cyanide has its uses, the search for safer alternatives is in full swing. Innovations in cyanide process technologies are emerging, with researchers exploring non-toxic substitutes that could revolutionize how we extract resources. From bioleaching to advanced filtration systems, the goal is to minimize environmental impact while maintaining efficacy—because who said we can’t have our cake and eat it too?
Conclusion and Future Perspectives
Summary of Key Points
As our journey through potassium cyanide’s tangled history shows, this chemical is a double-edged sword. Once a weapon of war and crime, its role has transitioned into industrial applications with strict regulatory oversight. However, the shadows of its past linger, reminding us of the need for vigilance in handling and legislation.
Potential Developments in Chemical Safety and Regulation
Looking ahead, we can expect an ongoing evolution in the way potassium cyanide is regulated and utilized. As awareness of chemical safety continues to grow, future regulations may push for even stricter controls and innovations in safer alternatives. It’s a brave new world, and with it comes not just responsibility, but also the potential for groundbreaking advancements that could revolutionize not only the chemical industry but our approach to safety and environmental stewardship.In conclusion, potassium cyanide’s journey from its early discoveries to contemporary applications highlights both its utility and the significant risks associated with its use. As industries continue to evolve and safety regulations become more stringent, the importance of understanding this chemical compound remains paramount. Continued research and innovation are essential in ensuring that potassium cyanide can be managed safely while minimizing its potential hazards. Ultimately, striking a balance between its beneficial applications and the inherent dangers will be crucial for its future in science and industry.
FAQ
What are the primary uses of potassium cyanide today?
Potassium cyanide is primarily used in gold extraction, electroplating, and organic synthesis in the pharmaceutical industry.
Why is potassium cyanide considered highly toxic?
Potassium cyanide is highly toxic because it inhibits cellular respiration by blocking the use of oxygen in the body, leading to potentially fatal consequences if ingested or inhaled.
What safety regulations exist for handling potassium cyanide?
Safety regulations for handling potassium cyanide include strict labeling requirements, mandatory safety training for workers, and the implementation of personal protective equipment (PPE) to minimize exposure risks.
Are there alternatives to potassium cyanide in industrial applications?
Yes, there are several alternatives being researched and developed, such as thiosulfate and other less toxic compounds, which can be used in gold extraction and other applications to reduce environmental and health risks.
Additional information
| POTASSIUM CYANIDE TABLETS | 1 box ( 20 tablets , 10mg each ), 2 boxes ( 40 tablets , 10mg each ), 3 boxes ( 60 tablets , 10mg each ) |
|---|